
Antigen Rapid Test
NANBEI Is Professional On Providing One-step Solution Of Laboratory Instruments And Equipment
Brand:NANBEI
Model:
Application:
Model: ICO-3000
For use with anterior nasal swab specimens
For in vitro Diagnostic Use Only
This product has been authorized by FDA under an Emergency Use Authorization (EUA)
PRODUCT DESCRIPTION
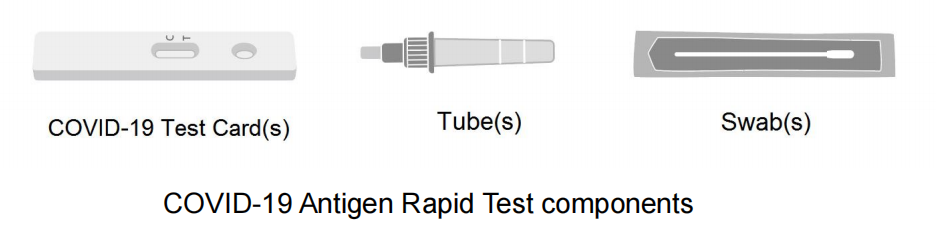
The COVID-19 Antigen Rapid Test requires the following elements for operation.

Materials provided in the Test Kit:
| Kit components | 2 tests Kit | 5 tests Kit | 40 tests Kit |
| COVID-19 Test Card(s) | 2 ea/box | 5 ea/box | 40 ea/box |
| Nasal Swab(s) | 2 ea/box | 5 ea/box | 40 ea/box |
| Tube(s) | 2 ea/box | 5 ea/box | 40 ea/box |
| Lay User Instruction for Use | 1 ea/box | 1 ea/box | 1 ea/box |
Materials required but are not provided in the kit:
• Smartphone (supplied by the user. iOS 12 or above. android 6.0 or above)
• User is required to download the “COVID-19 Antigen Rapid Test” App for iOS or Android phones. User should follow the step-by-step instructions in-app to complete the test.
PRINCIPLE OF PROCEDURES
The COVID-19 Antigen Rapid Test employs lateral flow immunoassay technology. Using this test allows for the rapid detection of nucleocapsid protein from SARS-CoV-2. To begin the test, a self-collected anterior nares swab samples in individuals aged 15 and older or individuals between the age of 2 to 14 a swab collected by a parent or guardian is inserted into the Tube. The liquid in tube interacts with the specimen and facilitates exposure of the appropriate viral antigens to the antibodies used in the test. The liquid in tube now containing the specimen is added to the Sample Port of the COVID-19 Test Card.
If the extracted specimen contains SARS-CoV-2 antigens, a pink-to-purple T Line, along with a pink-to-purple C Line will appear on the COVID-19 Test Card indicating a positive result. If SARS-CoV-2 antigens are not present, or present at very low levels, only a pink-to-purple C Line will appear.